NANOVIS® Corporate News
Nanovis’ FDA-Cleared Nanotechnology is Used to Enhance Medtronic PEEK Interbody Fusion Devices for Use in Spine Surgery
COLUMBIA CITY, Ind. April 19, 2022
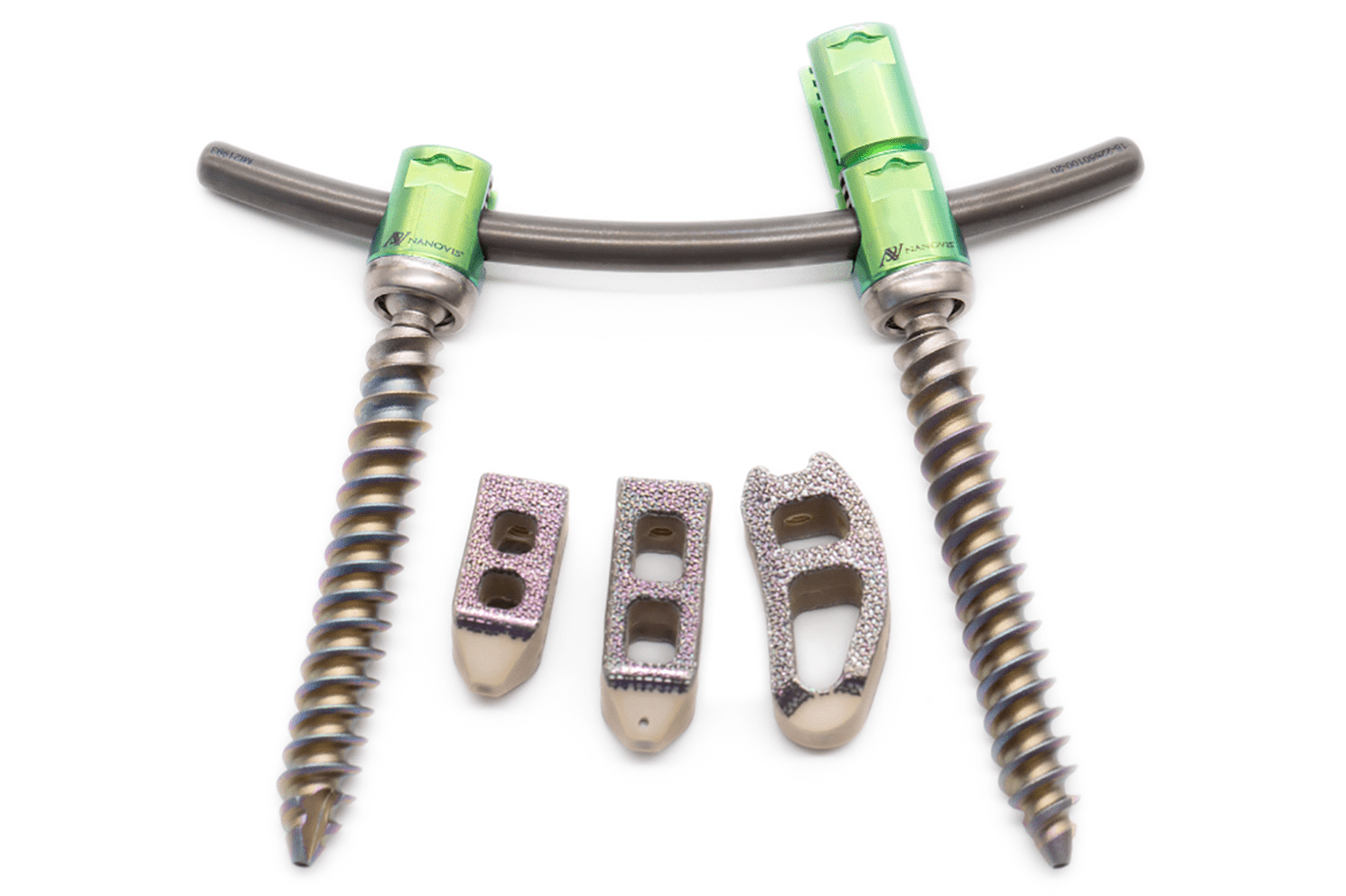
Nanovis, a technology-driven company committed to discovering unmet clinical needs, developing innovative and enabling solutions, and validating new technologies for clinical and market acceptance, announces subsidiary, Nanovis Spine, achieved record sales for FY 2021, driven by their best-in-class nanotechnology portfolio of interbodies and pedicle screws.
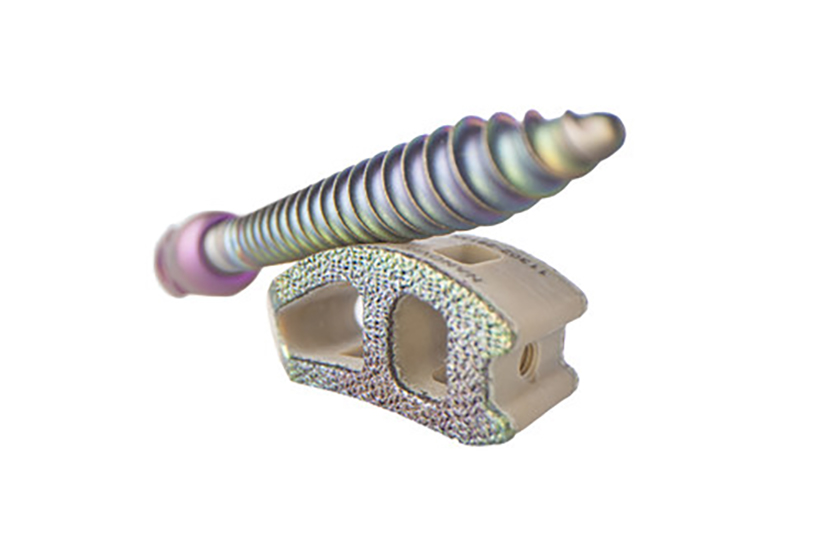
First use of a nanotechnology enhanced pedicle screw with label claims to increase and accelerate human osteoblast and mesenchymal stem cell calcified extracellular matrix production in vitro
Bioceramic Nanotube- Enhanced Nano FortiCore and New Customers Increase Sales.
Carmel, Indiana (July 14, 2020) – Nanovis, a unique technology company focused on nanotechnology for improving bone growth and fighting infection, today reported a record sales month in June 2020.
First Ever Pedicle Screw System with Nanotechnology Designation and Osteoblast and Stem cell data comparing performance to other surface types.
First Nanotechnology Designation for Bioceramic Nanotube Surface.
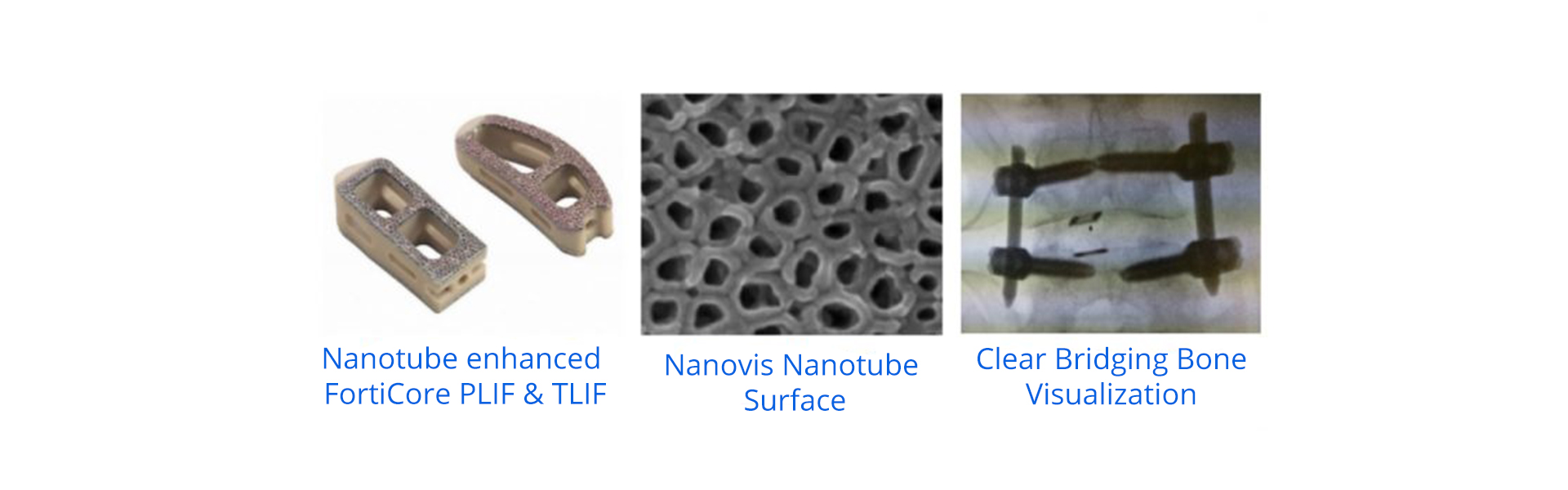
CARMEL, Indiana (Oct 22, 2019) – Nanovis today announced that it received the first 510(k) clearance for a bioceramic nanotube surface that demonstrated the FDA requirements for nanotechnology.
First use of novel bioceramic enhanced nanotube surface in the world.
CARMEL, Indiana. (July 30, 2019) – Nanovis today announced the commercial launch of its bioceramic nanotube enhanced FortiCore interbodies following a successful alpha launch.
The FortiCore interbodies are designed with a unique, proprietary, patent protected bio-ceramic enhanced titanium nanotube surface. The nanotubes are applied to a deeply porous, fully interconnected titanium scaffold intermolded with a PEEK core for preferred modulus and plain x-ray visualization.
Company recognized for its nanotechnology-based spinal devices
CARMEL, Indiana. (May 8, 2019) – Nanovis today announced that MedTech Outlook recognized Nanovis as a Top 10 Orthopedic Solution Provider, 2019. Its industry-leading fixation technologies offer surgeons and hospitals the best aspects of fixation, visualization, and durability. Nanovis’ developmental infection technology platforms promise to offer surgeons and hospitals much-needed bactericidal solutions.
Nanovis today announced that Global Health & Pharma magazine recognized Nanovis as the Best Nanotechnology Driven Implant Company, 2018.
CARMEL, Ind., November 14, 2018— Nanovis, a leader in nanomedicine for the spine, today announced adding 22mm and 25mm lengths to its FortiCore® rotatable Posterior Lumbar Interbody Fusion (PLIF) devices.
With over 5,000 FortiCore interbodies now implanted, Nanovis plans to further accelerate its technology driven growth with the expansion of their FortiCore PLIF product line with the addition of 22 mm and 25 mm lengths that can be rotated in situ for optimal placement.
This foundational nanotechnology patent, covering the use of ceramics on spinal implants with nanopores, builds on Nanovis’ superior portfolio of fixation technologies.
CARMEL, Indiana. – Sept. 11, 2018 – Nanovis, a leader in nanomedicine for the spine, today announced a licensing agreement with the University of Nevada, Reno for a key nanosurface technology patent covering the use of ceramics on implants with nanopores.
This foundational patent allows development of ceramics for medical implants with nanosurfacing that enhances cell binding and drug delivery depending on the purpose of implantation.
This funding supports surging demand from surgeons and distributors for Nanovis’ nano-technology enhanced spinal implants.
Carmel, Ind. (Aug. 21, 2018) – Nanovis, an innovative and fast-growing technology company selling nano-technology enhanced spinal implants, announced today the successful completion of a $5.5 million funding round brokered by Commenda Securities. Key investors include Elevate Ventures, 1st Source Capital Corporation, Purdue’s Foundry Investment Fund, Commenda Capital, and Ellipsis Ventures.
Spinal Implants Cleared with the Most Advanced Nanosurface and Best Imaging
Carmel, Ind. (March 28, 2018) – Nanovis, today announced the successful FDA clearance of its FortiCore® TLIF and PLIF interbodies featuring a Nanosurface-enhanced deeply porous titanium scaffold intermolded with a PEEK core.
Nanovis’ Deeply Porous Spinal Implants Show Favorable Clinical Adoption.
Carmel and Columbia City, Ind. (February 16, 2017) – Nanovis, a life sciences company committed to developing implant systems that reduce fixation related complications and infections, today announced the successful alpha launch of its FortiCore® PLIF featuring a deeply porous titanium scaffold interdigitated with a PEEK core, and implantation of the 2,000th FortiCore® implant.
Nanovis’ Deeply Porous Spinal Implants with Nanotube-Enhanced Surfaces May Significantly Improve Patient Recovery from Spinal Fusion Procedures
Carmel, Ind. (March 8, 2016) – Nanovis, a life sciences company committed to developing scientifically advanced regenerative platforms for implantable medical devices, today announced a grant award from the National Institute on Aging, part of the National Institutes of Health (NIH).
Proprietary Approach Layers Titanium Scaffold Surface Technology on PEEK Implants Creating Differentiated Devices
CARMEL, Ind., Oct. 15, 2015 /PRNewswire/ — Nanovis, a life sciences company committed to developing scientifically advanced regenerative platforms for implantable medical devices, today announced the availability of the FortiCore® wedge shaped lordotic cervical cage and a transforaminal lumbar interbody fusion (TLIF) device with increased scaffolding.
Combination of a deeply porous titanium scaffold with a PEEK center and advanced nanotechnology may reduce healing and fixation times
Carmel, Ind. (September 22, 2015) – Nanovis Spine today announced the award of a significant research grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (NIH).
Carmel, Ind. (June 18, 2015) – Nanovis Spine, LLC (Nanovis) announced today the launch of the company’s FortiBridge® cervical plate system. FortiBridge cervical plates allow for high angulation screw placement with a smooth, esophagus-friendly profile. The FortiBridge cervical plate system offers a full range of short and long sizes in either steam sterilizable or individually sterile packaging formats.
Carmel, Ind. (September 22, ) – Nanovis Spine, LLC (Nanovis) announced today that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance of the company’s sterile packed FortiCore® interbody fusion devices. Unlike conventional implants comprising PEEK or sprayed titanium coating, FortiCore® implants have the benefits of a highly porous titanium scaffold engineered for strong, durable integration with a PEEK core.

















Connect With Us
Columbia City, IN 46725
United States
Recent News