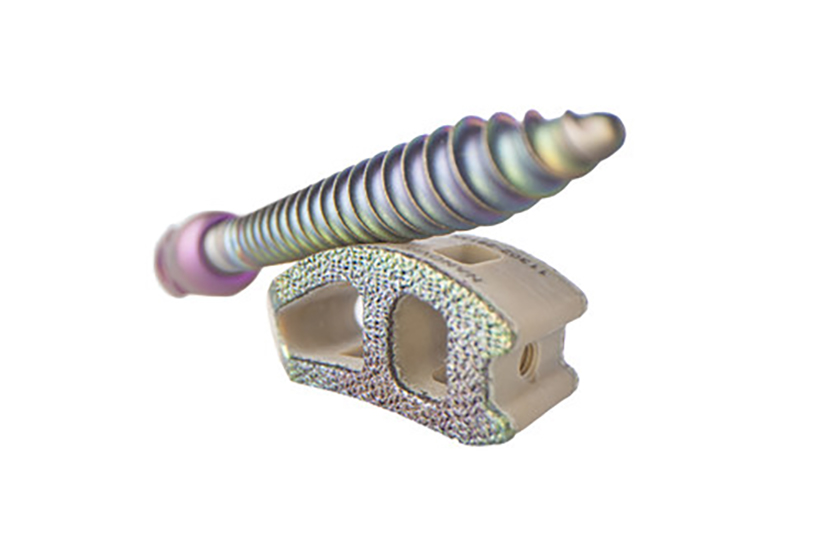
Nanovis Announces Alpha Launch of the First and Only Bioceramic Nanotube-Enhanced Pedicle Screw System, Nano FortiFix®
First use of a nanotechnology enhanced pedicle screw with label claims to increase and accelerate human osteoblast and mesenchymal stem cell calcified extracellular matrix production in vitro
CARMEL, Ind., Sept. 28, 2021 - Nanovis, an award winning technology company with unique and differentiated nanotechnology surfaces for improving bone growth and fighting infection, is pleased to announce the first implantations of its Bioceramic Nanotube-Enhanced pedicle screw system, Nano FortiFix, the only nanotechnology designated titanium-surfaced pedicle screw.
Nano FortiFix pedicle screws are designed with a proprietary and patent protected bio-ceramic nanotube surface. Unlike other technologies, Nanovis's bioceramic nanotubes were granted a nanotechnology designation with specific label language stating "The nanotube arrays have been shown to increase and accelerate calcified extracellular matrix production in vitro". The Nano FortiFix label also includes statistically significantly superior human osteoblast and mesenchymal stem cell calcified extracellular matrix production data compared to other surfaces.
"This nanotechnology label and accompanying comparative data provides unusual clarity for surgeons and hospital contracting executives when selecting a technology that is unequivocally differentiated for their patients seeking the best care," says Matt Hedrick, Nanovis CEO.
Nanovis was the first company to receive nanotechnology designation for PEEK enhanced titanium interbodies and is the first and only company to receive nanotechnology designation for titanium-surfaced pedicle screws.
"I'm pleased to now have a pedicle screw system with cutting edge nanotechnology to offer my patients. I've been using Nano FortiFix pedicle screws in combination with Nano FortiCore interbodies, giving my patients a fusion using 360˚ nanotechnology. It's exciting that nanotechnology is no longer limited to the interbody space," says Alan McGee MD of Parkview Health in Fort Wayne, IN.
"Nanovis is pleased with the success of this alpha launch. The feedback from innovative and discerning surgeons and distributors has been positive, encouraging, and primes us for the full commercial launch of our open and MIS systems," says Jeff Shepherd, Nanovis Vice President of Sales.
This is Nanovis' 5th implant system cleared with a bioceramic nanotube surface establishing a well understood regulatory pathway. Nanovis' commitment to help reduce the pain and suffering from implant loosening has never been higher and we are grateful to the surgeons, scientists, and employees that have created such a powerful combination of technology-enhanced systems.
Nanovis is actively expanding distribution for bioceramic nanotube enhanced Nano FortiCore interbodies and Nano FortiFix pedicle screws. To discuss opportunities, please contact Jeff Shepherd, Vice President of Sales, at
About Nanovis
Nanovis is a unique technology company that discovers, develops, and commercializes innovative nanotechnology platforms designed for improving bone growth and fighting infection. Nanovis' bioceramic nanotube surface is FDA cleared with a differentiated label, is well-proven and is ready to quickly upgrade any orthopedic implant made of titanium or titanium alloy. Nanovis' Nano FortiCore interbodies also utilize a deeply porous titanium scaffold (licensed from Sites Medical). Nanovis' Nano FortiFix pedicle screws (licensed from Altus) utilize Nanovis' Bioceramic Nanotube surface. Nanovis's advanced bioceramic bactericidal nanotube surface technology is in pre-clinical studies, and Nanovis's breakthrough localized infection technology targeting multi drug resistant bacteria is in pre-clinical studies.
If you would like more information about Nanovis, please contact:
Jeff Shepherd
Nanovis, LLC
(317) 432-1342
SOURCE Nanovis



Connect With Us
Columbia City, IN 46725
United States
Recent News